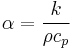Thermal diffusivity
In heat transfer analysis, thermal diffusivity (usually denoted α but a, κ, k, and D are also used) is the thermal conductivity divided by density and specific heat capacity at constant pressure.[1] It has the SI unit of m²/s. The formula is:
where
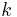 is thermal conductivity (W/(m·K))
is thermal conductivity (W/(m·K))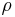 is density (kg/m³)
is density (kg/m³)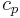 is specific heat capacity (J/(kg·K))
is specific heat capacity (J/(kg·K))
The denominator 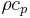 can be considered the volumetric heat capacity (J/(m³·K)).
can be considered the volumetric heat capacity (J/(m³·K)).
In a sense, thermal diffusivity is the measure of thermal inertia.[2] In a substance with high thermal diffusivity, heat moves rapidly through because the substance conducts heat quickly relative to its volumetric heat capacity or 'thermal bulk'. The substance generally does not require much energy from its surroundings to reach thermal equilibrium.
Thermal diffusivity is often measured with the flash method. It involves heating a cylindrical sample with a short energy pulse at one end and analyzing the temperature change at the other end.[3]
| Material | Thermal diffusivity (m²/s) |
Thermal diffusivity (mm²/s) |
|---|---|---|
| Pyrolytic graphite, parallel to layers | 1.22 × 10−3 | 1220 |
| Silver, pure (99.9%) | 1.6563 × 10−4 | 165.63 |
| Gold | 1.27 × 10−4 [5] | 127. |
| Copper | 1.1234 × 10−4 | 112.34 |
| Aluminium | 8.418 × 10−5 | 84.18 |
| Aluminum 6061-T6 Alloy | 6.4 × 10−5 [5] | 64. |
| Water vapour (1 atm, 400 K) | 2.338 × 10−5 | 23.38 |
| Air (1 atm, 300 K) | 2.2160 × 10−5 | 22.16 |
| Aluminium oxide (polycrystalline) | 1.20 × 10−5 | 12.0 |
| Steel, 1% carbon | 1.172 × 10−5 | 11.72 |
| Steel, stainless 304A | 4.2 × 10−6 [5] | 4.2 |
| Iron | 2.3 × 10−5 [5] | 23. |
| Silicon | 8.8 × 10−5 [5] | 88 |
| Quartz | 1.4 × 10−6 [5] | 1.4 |
| Silicon Dioxide (Polycrystalline) | 8.3 × 10−7 [5] | 0.83 |
| Water (300 K) | 1.4 × 10−7 [5] | 0.14 |
| Polyvinyl Chloride (PVC) | 8 × 10−8 [5] | 0.08 |
| Alcohol | 7 × 10−8 [5] | 0.07 |
| Air | 1.9 × 10−5 [5] | 19 |
| Argon (300 K, 1 atm) | 2.2×10−5[6] | 22 |
| Helium (300 K, 1 atm) | 1.9×10−4[6] | 190 |
| Hydrogen (300 K, 1 atm) | 1.6×10−4[6] | 160 |
| Nitrogen (300 K, 1 atm) | 2.2×10−5[6] | 22 |
| Pyrolytic graphite, normal to layers | 3.6 × 10−6 | 3.6 |
| Sandstone | 1.12–1.19 × 10−6 | 1.15 |
| Tin | 4.0 × 10−5 [5] | 40. |
| Brick, common | 5.2 × 10−7 | 0.52 |
| Glass, window | 3.4 × 10−7 | 0.34 |
| Rubber | 1.3 × 10−7 | 0.13 |
| Nylon | 9 × 10−8 | 0.09 |
| Wood (Yellow Pine) | 8.2 × 10−8 | 0.082 |
| Oil, engine (saturated liquid, 100 °C) | 7.38 × 10−8 | 0.0738 |
See also
References
- ^ Lide, David R. (2009). Handbook of Chemistry and Physics (90 ed.). Boca Raton, Florida: CRC Press. p. 2-65. ISBN 978-1-4200-9084-0.
- ^ Venkanna, B.K. (2010). Fundamentals of Heat and Mass Transfer. New Delhi: PHI Learning. p. 38. ISBN 978-81-203-4031-2. http://books.google.com/books?id=IIIVHRirRgEC&pg=PA38. Retrieved 1 December 2011.
- ^ Thermitus, M.-A. (October 2010). "New Beam Size Correction for Thermal Diffusivity Measurement with the Flash Method". In Gaal, Daniela S.; Gaal, Peter S. (eds.). Thermal Conductivity 30/Thermal Expansion 18. 30th International Thermal Conductivity Conference/18th International Thermal Expansion Symposium. Lancaster, PA: DEStech Publications. p. 217. ISBN 978-1-60595-015-0. http://books.google.com/books?id=F9row3bxLuYC&pg=PA217. Retrieved 1 December 2011.
- ^ Brown; Marco (1958). Introduction to Heat Transfer (3rd ed.). McGraw-Hill. and Eckert; Drake (1959). Heat and Mass Transfer. McGraw-Hill. ISBN 0891165533. cited in Holman, J.P. (2002). Heat Transfer (9th ed.). McGraw-Hill. ISBN 0070296391.
- ^ a b c d e f g h i j k l Jim Wilson (August 2007). Materials Data. http://www.electronics-cooling.com/2007/08/thermal-diffusivity/.
- ^ a b c d Lide, David R., ed (1992). CDC Handbook of Chemistry and Physics (71st ed.). Boston: Chemical Rubber Publishing Company. cited in Baierlein, Ralph (1999). Thermal Physics. Cambridge, UK: Cambridge University Press. p. 372. ISBN 0-521-59082-5. http://books.google.com/books?id=fqUU71spbZYC&pg=PA372. Retrieved 1 December 2011.